Treatment for the elimination of dissolved ions in water through ion exchange resins that are reactivated through the use of regenerants.
Ion exchange involves the exchange of ions between a solid and a liquid, in which no substantial change occurs in the structure of the solid.
Since ion exchange is a reversible reaction, the exchange material can be regenerated for new processes.
Main types:
Technologies
ION EXCHANGE
- Demineralization
- Cation – Anion
- Mixed bed
- Denitrification
- Decarbonation
- Decalcification
- Others
APPLICATIONS
- As a post-treatment for other processes, e.g., reverse osmosis
- In industrial processes, associated with various sectors:
- In the agri-food industry
- In the chemical and pharmaceutical industry
- In the energy, electronics, and nuclear industries
- As surface treatment
- As feed water for boilers
- For water potabilization (removal of perchlorate and uranium, among other things)

OPERATION
Ion exchangers are insoluble substances in granular form, like small beads, called resins, which are capable of absorbing ions from a solution and in exchange yielding an equivalent amount of another ion without any apparent change in their physical appearance or solubility.
This exchange only works between ions of equal electrical charge (cations for cations and anions for anions).
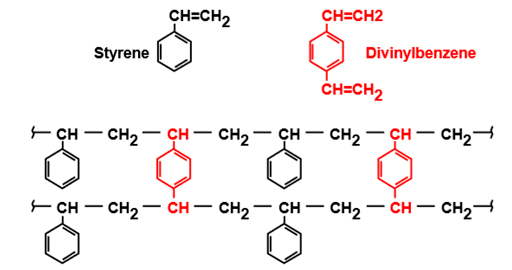
The most common resin is obtained by copolymerization of styrene and divenylbenzene (DVB), which solidify in the form of spheres into which the active exchange group is inserted.
These resins will have different specific surface area and uniformity depending on the type of application and the active group of radicals.
CATIONICS(C) |
STRONG (SC) Sulfonic groups (R-SO3) |
Regenerated with NaCl: Exchange of divalent and trivalent cations for sodium ions. Regenerated with strong acid: Exchange of all types of cations for H+ |
|
WEAK (WC) Carboxylic groups (R-COOH) |
Regenerated with strong acid: Weak acid and hydroxide cations are exchanged for H+ | |
|
ANIONICS (A) |
STRONG (SA) Quaternary ammonium groups |
Exchange strong and weak acid anions (SiO2 , CO2, organic acids) for OH- groups |
|
WEAK BASIC (WA) Tertiary ammonium groups |
Exchange strong acid anions (Cl-, SO4=, NO3) for OH- groups | |
| MODERATE BASIC | Mix of the two previous groups | |
| special | Macroporus | Can be SC, WC, SA, WA and have higher mechanical, temperature, and oxidant resistance |
| Metal selective | SC regenerated with acid | |
| Scravanger o adsorbents | Microporous SA, retain organic matter and colloids. Regenerate with NaCl |
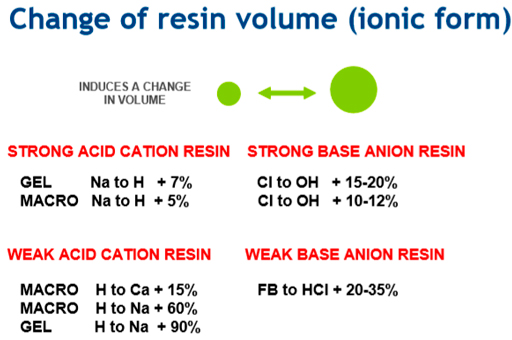
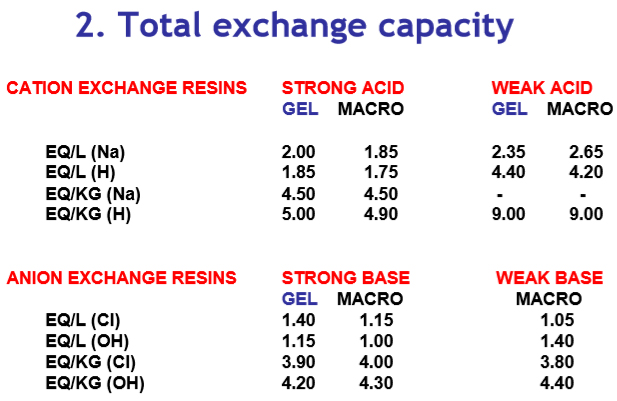
The exchange capacity of the resins will be different for each type. This is a measure of the total number of active positions that are available for exchange (eq/L or eq/kg). In addition, a change in volume is induced.
Typical values for resins in different ionic forms:
Once the exchange capacity is reached, we can say that the resin is exhausted.
When this happens, it proceeds to be regenerated. This is a set of operations that allows the displacement of the retained ions until the resin is returned to its initial charge state.
The first operation of the process is backwashing: the water is passed upwards, causing the decompaction of the bed and the elimination of possible particles that may have been retained.
This operation is performed at a critical speed set by the resin manufacturer.
Then, in the washing direction, a regenerating solution will be introduced, which will contain:
- HCL or HSO4 for cation exchange
- NaOH (sodium hydroxide) for anionic exchange
This operation is performed at a specific controlled concentration in each case.
Next comes the washing of the bed, which consists of two stages: a slow wash to ensure that the regenerant solution reaches the entire bed, and a quick wash to remove any remaining regenerant solution and leave the resin in the ideal state to start a new work cycle.
The process that occurs during regeneration is the reverse of that of depletion.
- For cation resins: Na-R + HCl -> H-R + NaCl
- For anionic resins: Cl-R + NaOH -> R-OH + NaCl
Natural waters contain calcium and magnesium ions that form poorly soluble salts.
These cations, among other less common ones such as strontium or barium, are called hardness ions.
When water evaporates, they can precipitate and form solids.
Hard water produces scale and can generate turbidity, so it is necessary to eliminate these components as much as possible to achieve softer water.
For this purpose, strong acidic cation exchange resins are used in which the exchangeable ion is sodium Na, generically called cation resins in the sodium cycle.
The general exchange reaction in the softening process that takes place can be expressed by:
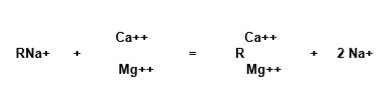
Sodium cycling resin (RNa) comes in contact with calcium and magnesium ions (Ca2+, Mg2+) and retains them, releasing sodium cations into the water.
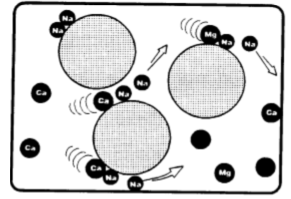
The sodium cations released by the resin bind to the anions to which the calcium and magnesium were bound, forming the same salt in sodium form. In the case of calcium and magnesium bicarbonates, we see:
In softening or descaling, the salinity of the treated water is the same as that of the water to be treated, with the difference being that the salts that contained the hardness-causing ions are transformed into highly soluble sodium salts that do not cause scaling.
For the resin regeneration process, a brine solution (sodium chloride) is used:
- Ca-R + 2NaCl -> CaCl2 + Na2 R